Once you have sterile agar plates poured, cooled, and hardened, what do you do with them? This depends on what goals you have in mind. To start out with I would like to caution those working with bacteria against the very shiny colonies. These are generally encapsulated bacteria. They have a thick gelatinous coating on them that helps protect their cell walls from attacking antibodies. As a result they are much better suited for living within the human body than are unencapsulated bacteria. Essentially what this means is that the encapsulated bacteria tend to be pathogenic more often than unencapsulated bacteria. Be ware of colonies of bacteria with a thick glossy look to them. When in doubt, destroy them. It isn't worth the risk. Also refrain from taking cultures from ill people. It makes no sense for amateurs to try cultivating dangerous species.
The first thing to do is build a few tools to help you work with your agar. Depending on what you want to do, you may need an innoculation loupe, an innoculating needle, a good set of tweezers, hook, and perhaps a scapel. Tweezers are probably best bought at any store with a cosmetics department. Any sort that feels comfortable to the hand should be fine. Tweezers are the least useful tool on this list anyway.
Much more important is the loupe and the needle. Both of these may be made from 16 or 18 gauge wire. Use wire cutters to snip the end of the wire off with as sharp a point on the wire as possible. Use a set of pliers to push this sharp point into the end of a pencil sized stick. Snip the wire off so that you now have a tool that looks like a pencil with a 4 to 5 inch wire sticking out of where the pencil's lead would normally be. Now in the case of the needle, simply use the wire cutter to snip the end of the wire so that it is as sharp as possible. Be careful, as this will be quite sharp in most cases. For the loupe, rather than sharpening the point, make sure it is cut off as bluntly as possible. Not curl the end back around to make a small circle at the end of the wire. Try to get the wire circle to about 1/4 inch outside diameter. If you are using needle nose pliers, the right size loupe will probably be about as small as you can make it. Try to wrap the end of the wire back around the stem part, to make the loupe more secure. If you can't get it, don't sweat it. It won't have to withstand much stress anyway. Commercial loupes and needles are only a few dollars and if you stick with your hobby you may find it worth the money to purchase them. For the time being you are just as well off with the free ones you make yourself. The hook is very similiar to the loupe, except the wire is only bent in a 180 degree curve. This allows for a hooking or snagging of materials to be transfered from one plate to another.
The scapel is perhaps the most difficult thing to improvise. Fortunately there are many small craft and hobby knives that will suffice. When picking one out, keep in mind that an easy to clean and sterilize knife will be much more useful than one that is difficult to keep clean. Wood and metal are easy to flame. Plastics may melt.
While it won't be used in direct contact with the agar, and alcohol lamp is an invaluable addition to your home lab. This can be had cheaply from a scientific supply store, and perhaps the local craft store. The can also be made from a small jar with a thin metal lid. Canning jars work well for this, as do small jelly jars. Use a nail to make a small hole in the lid, and gently thread some thick string or absorbent paper though the hole. Let a length of this wick material hang down to the bottom of the jar. Fill the jar will either rubbing alcohol or denatured alcohol, available from pharmacies and hardware stores. You may have to play with the size of the hole in the lid, and the amount of wick extending through it to achieve the desired effect. With either a home made alcohol lamp, or a commercial one, care must be taken to prevent a fire hazard. Never leave the lamp lit and unattended. Keep all combustibles away from it. It is a very good idea to have a fire extinguisher handy, or at least a box of baking soda, which is good for extinguishing a wide variety of fires. Previously I've recommended using rubbing alcohol for both sanitization and as a lamp fuel. I have found however that denatured alcohol tends to burn better, and stay lit better than most pharmaceutical grades of rubbing alcohol. Thus I'd recommend the rubbing alcohol (generally 70% iso-propyl alcohol) for sanitization, and denatured alcohol (available from most hardware stores) as a lamp fuel.
Each time you touch your tools to the agar surface, they must be sterile, unless you are making a deliberate innoculation. The common method of quick sterilizing tools is called flaming the tool. One often used method is to simply pass the tool through an alcohol flame three times. Some people will recommend holding the tool in the flame until it is glowing red hot. In reality something somewhere in between these should work for you. I like to dip my tools in a small container of alcohol, and then touch the tool to the flame. When the alcohol is burned off, I can be reasonably certain that the tool is now sterile. Regardless of the method used, the tool must be allowed to cool before touching your culture. A hot tool touching bacteria or fungi quickly results in dead organisms. For light gauge wire tools, 4 to 5 seconds should be enough. Touching a section of sterile agar with the tool is a quick way to test the temperature of the tool. If the agar sizzles, it was still too hot. As testing your tools on the agar can carmelize a small spot of agar, possibly introduce contaminants, and requires an extra step in doing something where speed is critical to begin with, I would recommend against using this test on a regular basis. Only use it until you become comfortable gauging the time needed for your tools to cool down in your work area after using your method of flaming them.
If you are interested in the study of bacteriology the first thing you should do is grab a good book for the identification of genera and species. Hopefully you have a dissecting scope to study the colonies in detail. Cotton swaps are good for collecting samples, and so are Q-tips. Sterility isn't really a concern if you are just practising and teaching yourself the basics. However once you start looking for particular species, or if you want to be certain that what is growing came from the sample you took rather than an opportunistic contaminant of your collecting materials, you will need to use sterile swabs. Most pharmacies or home health care stores stock sterile Q-tips. The ones with the long wooden handles are very nice to use. Cotton balls can also be wrapped in aluminum foil and pressure cooked for 15 minutes at 10 or 15 PSI. After you have your swab ready, go find something to sample. If you have a specific specimen you are looking for, you should be armed with a general idea of its natural habitat, and where you might find it. If you are using sterile swabs, leave them covered in their wrapping until you are ready to use them. Take the swab and gently but firmly wipe the surface of whatever you are sampling. Wrap it back in its covering, if you wish to avoid other contaminants. Take this swab then and gently swab it across the surface of the petri dish. Set this aside to incubate for a few days. Ideally the temperature should be at least 70F. If arrangements can be made to increase this to the low to mid 70's, so much the better.
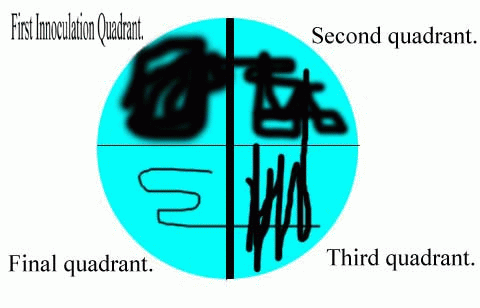
And alternative method of streaking the plate involves an innoculation loupe. Dab your swab in one corner of the petri dish. Take a loupe and flame it. After it cools, pass it once only across the surface of the petri dish where the swab has touched. Without lifting the loupe, drag it across to a previously untouched section of agar. Flame your loupe and do this again. If you view the dish as having 4 quarters, and you innoculate one quarter with the swab, and the other three corners by this method, then you have the right idea. Each quadrant is innoculated by a drag of the loupe from the neighboring quadrant. Usually the first quadrant will be totally covered with colonies of bacteria, and the other three quadrants will show decreasing numbers of colonies.
